-
00:00
1.
Western Blot - 2_Electrophoresis [Video from GeneTex]
-
00:19
2.
Clean the glass plates
-
00:32
3.
Assemble the gel apparatus
-
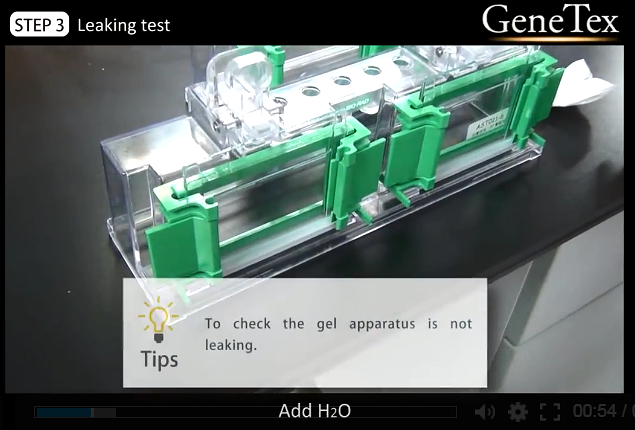
00:54
4.
Leaking test
-
01:03
4.1
Pour off H2O
-
01:11
5.
Preparation of separating gel
-
01:23
5.1
Add H2O
-
01:32
5.2
Add 1.5M Tris (pH8.8)
-
01:43
5.3
Add 30% polyacrylamide
-
01:54
5.4
Add 10% SDS
-
02:08
5.5
Add 10% ammonium persulfate (APS)
-
02:22
5.6
Add TEMED
-
02:40
5.7
Add the mixture into the gap between the glass plates
-
02:51
6.
Overlay with 75% EtOH
-
02:57
6.1
Remove 75% EtOH
-
02:57
6.2
Remove 75% EtOH
-
03:01
7.
Waiting for 30-60 min
-
03:05
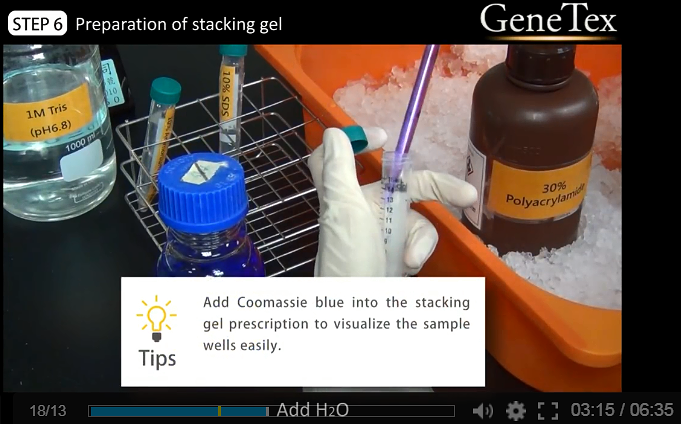
8.
Preparation of stacking gel
-
03:05
8.1
Add H2O
-
03:25
8.2
Add 1M Tris (pH6.8)
-
03:33
8.3
Add 30% polyacrylamide
-
03:45
8.4
Add 10% SDS
-
03:55
8.5
Add 10% ammonium persulfate (APS)
-
04:07
8.6
Add TEMED
-
04:23
8.7
Add the mixture into the gap between the glass plates
-
04:31
9.
Insert a clean comb into the stacking gel solution
-
04:47
10.
Waiting for 15-30 min
-
04:52
11.
Remove the comb gently
-
05:09
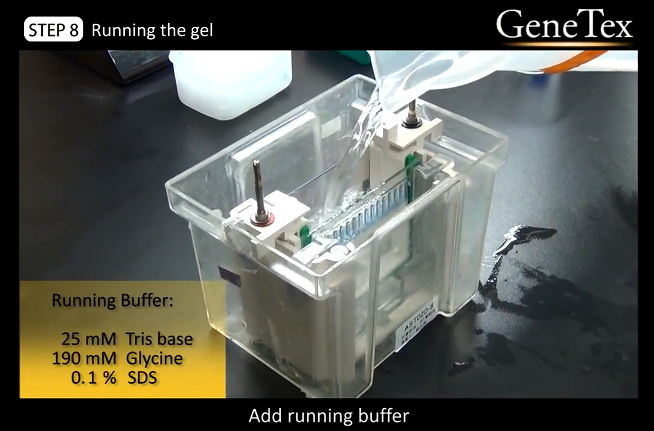
12.
Running the gel
-
05:09
12.1
Assemble the electrophoresis apparatus
-
05:29
12.2
Add running buffer
-
05:37
12.3
Vortex samples & protein marker
-
05:47
12.4
Centrifuge samples & protein marker
-
05:51
12.5
Loading samples & protein marker
-
06:07
12.6
Attach the electrophoresis apparatus to the power supply
-
06:12
12.7
Apply the voltage of 80V to the gel, run 15 min
-
06:18
12.8
After the dye front has moved into the stacking gel, increase the voltage to 140V and run 50 min
- 索引
- 筆記
- 討論
- 全螢幕
Western Blot - 2_Electrophoresis [Video from GeneTex]
長度: 06:35, 瀏覽: 1256, 最近修訂: 2018-12-19

播放影片: http://llai.cm.ntu.edu.tw/media/170
-
00:00
1.
Western Blot - 2_Electrophoresis [Video from GeneTex]
-
00:19
2.
Clean the glass plates
-
00:32
3.
Assemble the gel apparatus
-
00:54
4.
Leaking test
-
01:03
4.1
Pour off H2O
-
01:11
5.
Preparation of separating gel
-
01:23
5.1
Add H2O
-
01:32
5.2
Add 1.5M Tris (pH8.8)
-
01:43
5.3
Add 30% polyacrylamide
-
01:54
5.4
Add 10% SDS
-
02:08
5.5
Add 10% ammonium persulfate (APS)
-
02:22
5.6
Add TEMED
-
02:40
5.7
Add the mixture into the gap between the glass plates
-
02:51
6.
Overlay with 75% EtOH
-
02:57
6.1
Remove 75% EtOH
-
02:57
6.2
Remove 75% EtOH
-
03:01
7.
Waiting for 30-60 min
-
03:05
8.
Preparation of stacking gel
-
03:05
8.1
Add H2O
-
03:25
8.2
Add 1M Tris (pH6.8)
-
03:33
8.3
Add 30% polyacrylamide
-
03:45
8.4
Add 10% SDS
-
03:55
8.5
Add 10% ammonium persulfate (APS)
-
04:07
8.6
Add TEMED
-
04:23
8.7
Add the mixture into the gap between the glass plates
-
04:31
9.
Insert a clean comb into the stacking gel solution
-
04:47
10.
Waiting for 15-30 min
-
04:52
11.
Remove the comb gently
-
05:09
12.
Running the gel
-
05:09
12.1
Assemble the electrophoresis apparatus
-
05:29
12.2
Add running buffer
-
05:37
12.3
Vortex samples & protein marker
-
05:47
12.4
Centrifuge samples & protein marker
-
05:51
12.5
Loading samples & protein marker
-
06:07
12.6
Attach the electrophoresis apparatus to the power supply
-
06:12
12.7
Apply the voltage of 80V to the gel, run 15 min
-
06:18
12.8
After the dye front has moved into the stacking gel, increase the voltage to 140V and run 50 min
- 位置
-
- 資料夾名稱
- Western blot (Genetex版本)
- 發表人
- 賴亮全
- 單位
- 賴亮全教授
- 建立
- 2018-12-19 18:19:09
- 最近修訂
- 2018-12-19 23:32:42
- 長度
- 06:35
- 引用
- 1